|
|
|
|
|
|
The ozone layer has become a major topic of discussion among environmentalists, scientists and even the general public. Reports of holes in the ozone layer over Antarctica have raised considerable concern about environmental policy and have prompted many governments of the world to impose strict regulations regarding the release of ozone-depleting chemicals into the atmosphere.
Located in the upper atmosphere, the ozone layer is a blanket of ozone gas, O3, which until recently completely enveloped the earth and its lower atmosphere. Ozone is a highly unstable compound formed as a result of photochemical dissociation of the normal oxygen molecule, O2, which is in abundance and comprises about 21% of the earth's atmosphere. Ozone, on the other hand, makes up a very small percentage of the atmosphere -- less than one part in 1026 of all the oxygen in the atmosphere is present as ozone [2, p. 125]. As a matter of fact, if one were to take all of the ozone in the atmosphere at OoC and compress it to a pressure of 1 atmosphere, the thickness of the ozone layer over the earth's surface would be 0.34 cm on average [2, p. 123]. Thus, the ozone layer is a very reactive envelope of gas consisting of the fairly scarce O3 molecule.
The importance of ozone rests in its ability to absorb ultraviolet (UV) radiation, the component of sunlight that is harmful to life. UV radiation is light of short wavelength, less than 400 nanometers (nm). Radiation of short wavelength has very high amounts of energy associated with it. For example, UV radiation has enough energy to break the bonds of certain organic molecules, particularly the genetic material, DNA [2, p. 121], present in living tissues such as skin. In some cases the products of these photolyzed organic molecules are reactive fragments of molecules that can lead to the formation of various types of skin cancers. Sunburn is also the unpleasant result of prolonged exposure to UV radiation from the sun.
The ozone layer present in the atmosphere acts as a UV filter. The importance
of the ozone layer is that it effectively reduces the intensity of sunlight
in the range of 200 - 340 NM (the damaging UV radiation) while allowing the
passage of wavelengths of light in the 400 - 750 NM (the visible range of the
light spectrum). It has been hypothesized that higher forms of life could not
have evolved on earth until the protective ozone layer had developed [2,
p. 123].
Why is it then that we can still get sunburn if the ozone layer is filtering
out the UV rays? The ozone layer reduces the intensity of the UV light
but does not totally remove it. A fact about ozone molecules that will
become important in our analysis: They absorb radiation best in the 260 NM range
of wavelengths and are less efficient at absorbing radiation of other wavelengths.
Another question that frequently arises is why an ozone layer exists. Although this particular project focuses on locating the ozone layer, it helps to understand why the ozone layer exists where it does in the atmosphere. Our discussion here will focus on two of the main reactions involved in the production of ozone in the atmosphere.
The first reaction,
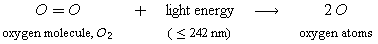
a photochemical reaction called photolysis, tells us why the ozone layer cannot exist close to the earth's surface or in the upper reaches of the atmosphere. This reaction shows that O atom formation is dependent upon light of the right energy and upon sufficient amounts of oxygen.
The second reaction shows how ozone is produced:
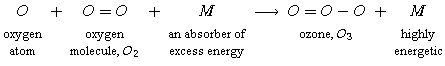
The oxygen atoms produced in the first equation are needed to react with oxygen molecules in the air in the presence of an energy absorber (such as N2) to produce ozone, O3.
At high altitudes the solar intensity is high, but the concentration of oxygen is too low to give large rates of O atom formation by photolysis. At low altitudes there is plenty of oxygen, but the intensity of radiation of the right wavelength that can dissociate the O2 molecule is too low. Somewhere in between, there is an area in the atmosphere where the rate of O atom production is a maximum [3, pp. 113-173].
|
|
|
| modules at math.duke.edu | Copyright CCP and the author(s), 1999 |